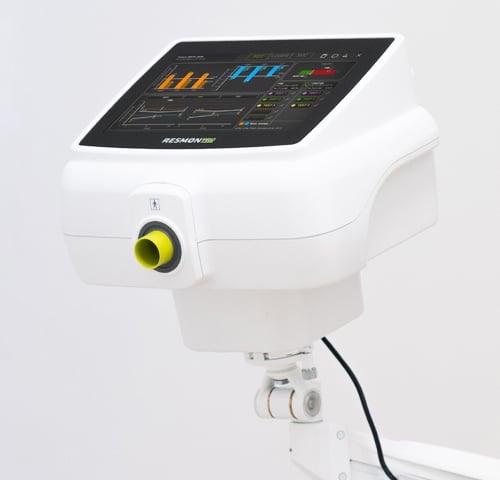
RESMON PRO FULL: the go-to solution for physicians seeking better diagnosis and staying on the cutting edge of innovation
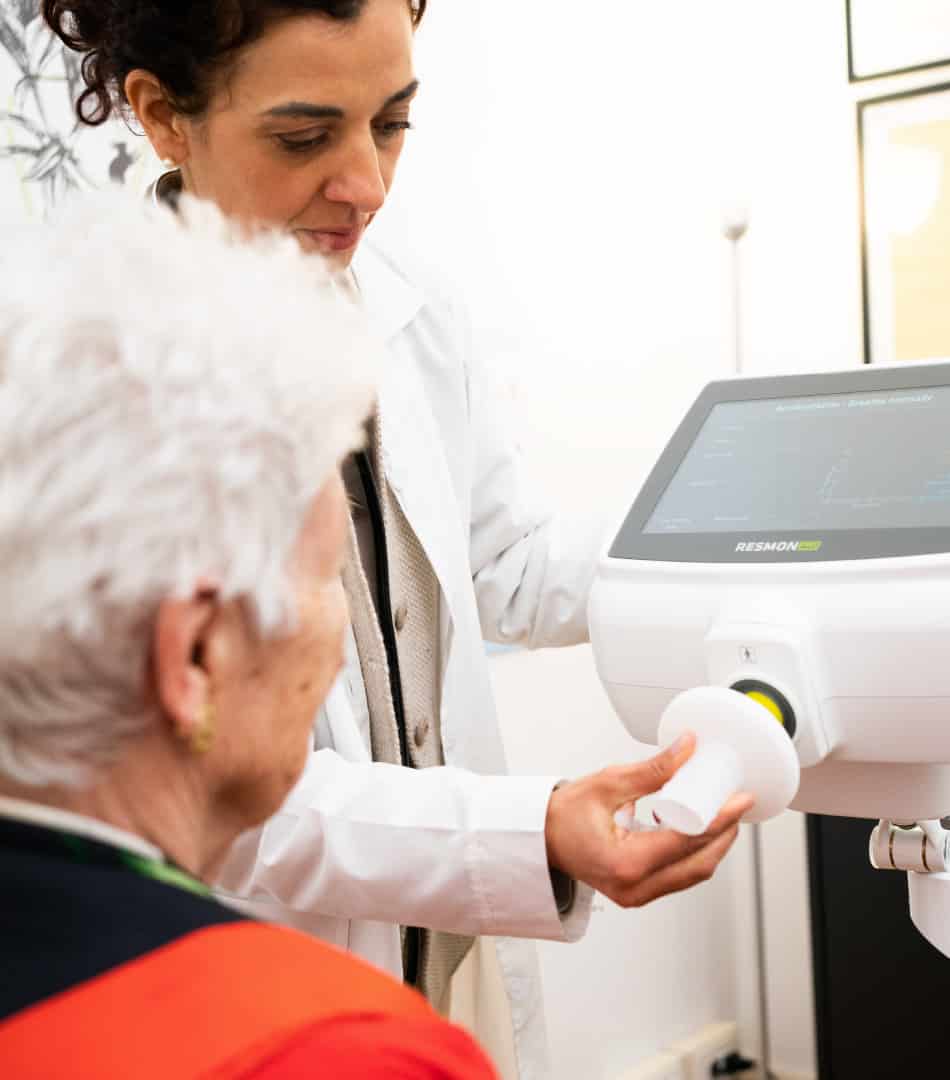
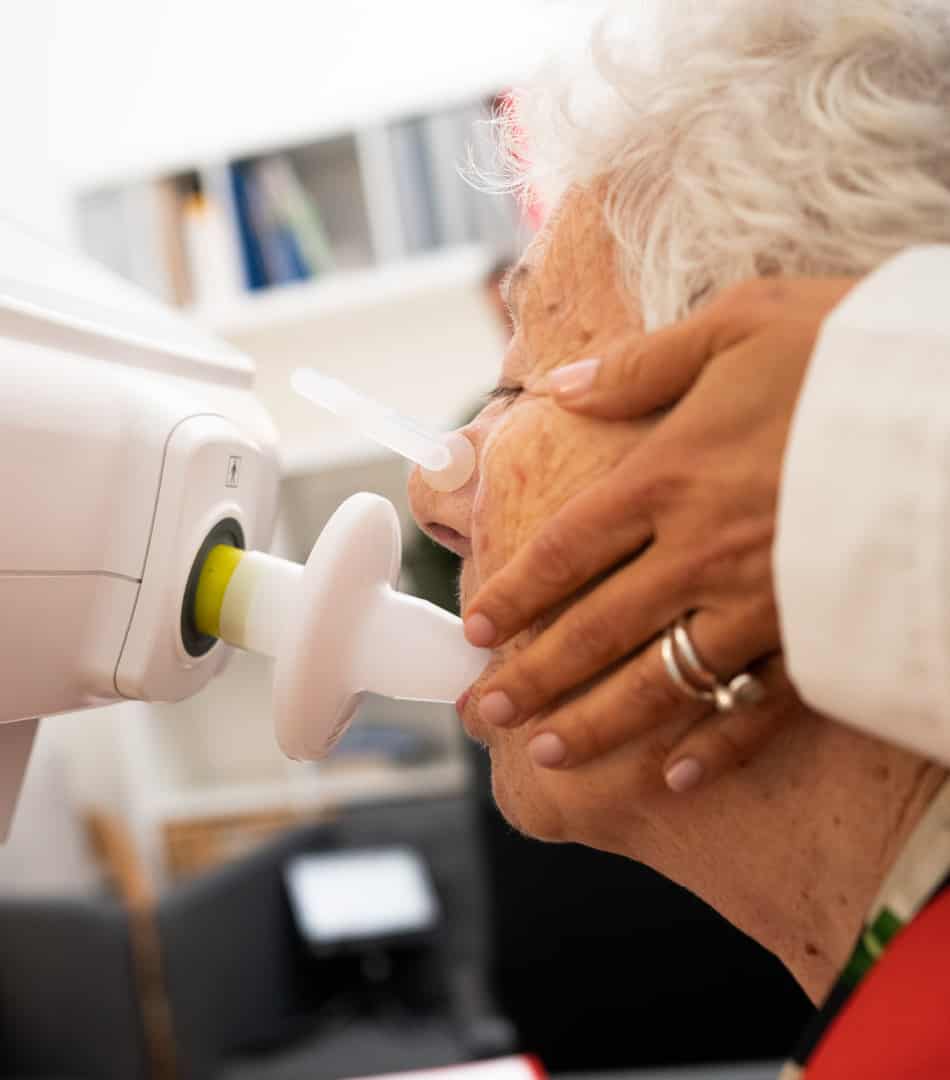
RESMON PRO FULL utilizes oscillometry (also known as forced oscillation technique or FOT) to accurately and non-invasively measure the mechanical properties of the respiratory system using just a few normal tidal breaths.
RESMON PRO FULL is a class IIa, CE-marked medical device (MDR 2017/745). Some of the described features may not be approved for use in your country/region. Please contact your local distributor for further information.
VERSATILITY FOR ALL PATIENTS
The versatility of RESMON PRO FULL extends to a variety of testing environments, from pulmonary laboratories to bedside assessments, private offices, and sites for clinical trials or research studies.
ADVANCED FEATURES FOR ENHANCED PERFORMANCE
RESMON PRO FULL is equipped with advanced calculation algorithms based on the latest scientific research and clinical findings, ensuring exceptional performance.
By integrating respiratory physiology with state-of-the-art technology, RESMON PRO FULL introduces a range of new parameters that combine oscillometry and static lung volume measurements, such as:
- oscillometric closing volume (CVfot) and critical reactance (Xcrit)
- oscillometric airway conductance (sGrs).
This comprehensive set of measurements enhances the physician’s ability to detect diseases, even in their earliest stages, and effectively monitor the impact of therapeutic interventions on disease progression.
DYNAMIC “WITHIN-BREATH” TESTING
- Real-time display of resistance (Rrs), reactance (Xrs) and flow or volume (user selectable)
- Measurement of inspiratory, expiratory and total Rrs and Xrs parameters
- Spectral analysis (spectrogram) with the calculation of R5-19, AX and Fres
- Automatic detection and quantification of tidal expiratory flow limitation (EFLt) with ΔXrs index graph and % of flow limited breaths (FL%), (patent nr. wo2003103493)
- Full respiratory pattern measurement and reporting of VE, VT, RR, Ti/Ttot, Vt/Ti, Vt/Te
- Customizable tidal loops graphs (available signals: Flow, Volume, Resistance, Reactance and Impedance)
SLOW VITAL CAPACITY TESTING
• SVC for monitoring of restrictive patterns
• IC for hyperinflation detection, pre-post testing for evaluating effect treatment
• Closing volume (CVfot) and critical reactance (Xcrit) for the evaluation of de-recruitment of peripheral airways
• Specific airway conductance (sGrs) for the evaluation of therapeutic intervention compensated for changes in lung volumes
THREE MEASUREMENT MODES
• Enhanced and optimized multi-frequency mode of 5-11-19 Hz, from children to adults
• Single-frequency mode options of 5, 6, 8, 10 Hz (for children, severely obstructed patients and special research purposes)
• Enhanced and optimized Pseudo Random Noise (PSN) of 5-37 Hz